|
SODIUM SULFITE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
7757-83-7 |
|
| EINECS NO. | 231-821-4 | |
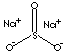
| FORMULA | Na2SO3 | |
| MOL WT. | 126.04 | |
|
H.S. CODE |
2832.10 | |
| TOXICITY | Oral mouse LD50: 820 mg/kg | |
| SYNONYMS | Sodium sulfite anhydrous; disodium sulfite; | |
| Natrii Sulphis; Natrium Sulfurosum; Natriumsulfit (German); sulfurous acid, disodium salt; exsiccated sodium sulfite; Sodium sulfite (2:1); Sulfurous acid, sodium salt (1:2); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
White crystalline powder |
|
| MELTING POINT |
Decomposes |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | 2.633 | |
| SOLUBILITY IN WATER | good | |
| pH | 9 | |
| VAPOR DENSITY | 4.35 | |
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 1 Flammability: 0 Reactivity: 0 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT |
|
|
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Sodium Sulfite is a White crystalline or powder; readily soluble in water, decomposes on heating. It is prepared from sulfur dioxide and sodium carbonate or caustic soda. It is a strong reducing agent and reacts with oxidants. It is primarily used in pulp and paper industry. It is used in water treatment as an oxygen scavenger agent , in the photographic industry to protect developer solutions from oxidation, in textile industry as a bleaching, as a desulfurizing and as a dechlorinating agent and in leather trade for the sulfitization of tanning extracts. It is used in chemical manufacturing as a sulfonation and sulfomethylation agent. It is used in the production of sodium thiosulfate. It is use in other applications include ore flotation, oil recovery, food preservative, making dyes, and detergent. It forms a bisulfite adduct with aldehyde and with ketones to a sulfonic acid. It is used to purify or isolate aldehydes and ketones. |
||
| SALES SPECIFICATION | ||
| TECH GRADE | ||
|
APPEARANCE |
White crystalline powder |
|
| Na2SO3 | 96.0% min | |
| SO2 |
48.8% min |
|
| IRON |
10ppm max |
|
| HEAVY METALS |
10ppm max |
|
| pH |
9.5 - 10.5 |
|
| FOOD GRADE | ||
|
APPEARANCE |
White crystalline powder |
|
| Na2SO3 | 98.0% min | |
| SO2 |
50.0% min |
|
| IRON |
5ppm max |
|
| ARSENIC |
0.5ppm max |
|
| HEAVY METALS |
10ppm max |
|
| pH |
9.5 - 10.5 |
|
| PHOTO GRADE | ||
|
APPEARANCE |
White crystalline powder |
|
| Na2SO3 | 97.5% min | |
| SO2 |
49.5% min |
|
| Na2S2O3 |
0.015% max |
|
| IRON |
5ppm max |
|
| HEAVY METALS |
10ppm max |
|
| pH |
9.5 - 10.5 |
|
|
TRANSPORTATION |
||
| PACKING | 25kgs , 1mt in Bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| BISULFITE ADDUCTS WITH ALDEHYDE GROUPS | ||
|
Acetone Sodium Bisulfite: [CAS RN: 540-92-1, (CH3)2C(OH)SO3Na] Crystals that have a slight sulfur dioxide odor and slightly fatty feel; freely soluble in water, decomposed by acids; used in textile dyeing and printing. Sodium Formaldehyde Bisulfite [CAS RN: 870-72-4 CH3NaO4S] A compound used as a fixing agent for fibers containing keratin, in metallurgy for flotation of lead-zinc ores, and in photography Indole-3-acetaldehyde Sodium Bisulfite [CAS RN: 20095-27-6, C10H9NO · NaHSO3]Glutaraldehyde bis(sodium bisulfite) [CAS RN: 7420-89-5, C5H12O8S2] used as an antiseptic Menadione Sodium Bisulfite [CAS RN: 57414-02-5, C11H9NaO5S] a water-soluble derivative of menadione provitamin which converted in the body to active vitamin K. Glyoxal sodium bisulfite [CAS RN: 332360-05-1, [CH(OH)SO3Na]2] nonaqueous form of glyoxal |
||
|
OTHER INFORMATION |
||
|
Sulfate (also spelled sulphate in Europe) is any chemical compound containing the SO42- ion related to sulfuric acid (H2SO4). Sulfates are salts or esters of sulfuric acid, formed by replacing one or both of the hydrogens with a metal or a radical as in sodium sulfate, Na2SO4. Sulfates in which both hydrogens are replaced are called normal sulfates. Bisulfate is a compound that has the HSO4- radical. Bisulfate (called also hydrogen sulfate or acid sulfate) is a compound formed by replacing only one hydrogen in sulfuric acid. Sulfite (also sulphite) is a compound that contain the sulfite ion SO32-. Sulfites are salts or esters of sulfurous acid (H2SO3), formed by replacing one or both of the hydrogens with a metal or a radical as in sodium sulfite, Na2SO3. Sulfites in which both hydrogens are replaced are called normal sulfites. Bisulfite is a compound that has the HSO3- radical. Bisulfate (called also hydrogen sulfite or acid sulfite) is a compound formed by replacing only one hydrogen in sulfurous acid. The term of 'meta' or 'pyro' is the chemical prefix for oxo acid formed through the loss of one water molecule (dehydration) from two molecules of ortho acid by heating. Pyrosulfuric acid is an example ( 2H2SO4 - H2O = H2S2O7). Ortho acid is the compound fully hydrated acid or its salts. Orthophosphoric acid is an example (2·H3PO4 = P2O5.3H2O), in contrast to the less hydrated form, pyrophosphoric acid (2·HPO3 = P2O5.H2O). Na2O5S2 is called sodium metabisulfite (2·HNaO3S - H2O). Sulfide is a compound having one or more sulfur atoms in which the sulfur is connected directly to a carbon, metal, or other nonoxygen atom; for example sodium sulfide, Na2S. Sulfide ion is S2- with oxidation number -2. Bisulfide ion is an anion formed by two sulfur atoms having an overall -2 charge, (S2)2-. Sulfamate is a salt of sulfamic acid (HSO3NH2). Calcium sulfamate Ca(SO3NH2)2 is an example. |
||